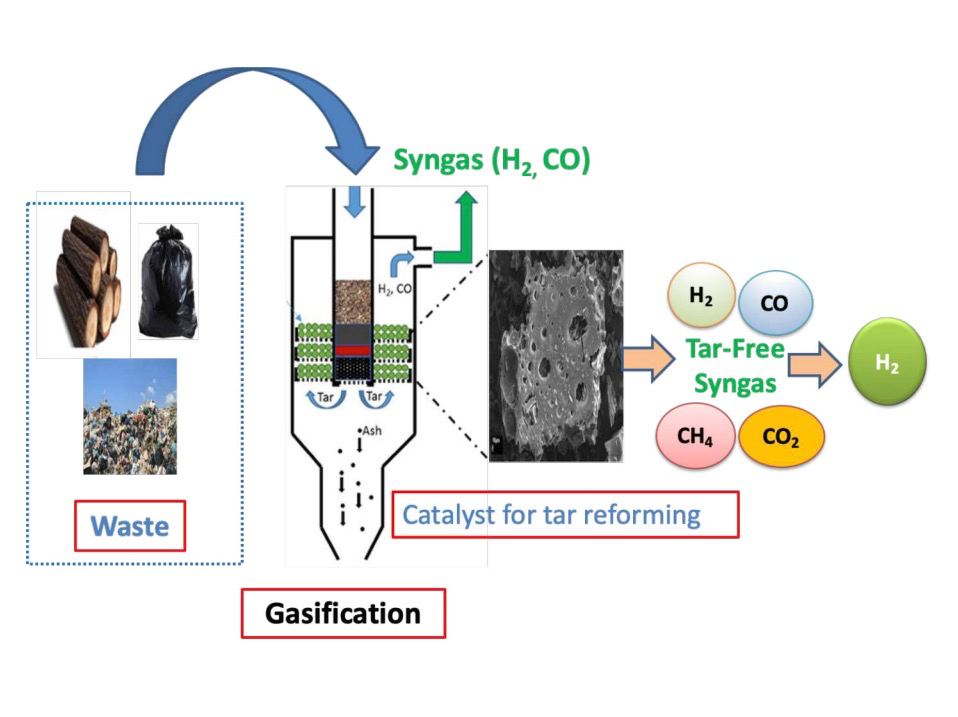
This project addresses issues associated with energy consumption and waste generation as they are expected to increase worldwide as a result of growths in population and industrialization. Total worldwide energy demand is expected to be about 25% higher in 2040 compared to the energy consumption in 2014, and fossil fuels will still continue to dominate the global energy use. The major source of energy comes from fossil fuels and accounts for 85% of total energy use and where oil remains the world’s largest source of energy. Therefore, managing the waste in a sustainable way and developing waste to energy facilities would help to solve both issues. Recovering energy from waste is performed through thermal treatment processes such as gasification, incineration and pyrolysis. Automobile tires are a complex mixture of very different materials, which include several rubbers, carbon blacks, steel cord and other organic and inorganic minor components. This complex nature of used car tires makes it difficult to recycle them. The main component of tires rubber is a chemically cross-linked polymer and therefore is neither fusible nor soluble and consequently cannot be remolded into other shapes without serious degradation. Tire pyrolysis/gasification for hydrogen and fuel production at present is an interesting and challenging area of research. The proposed project offers a technology for a renewable energy production. Most of previous studies focus on gasification of wastes using a commercial catalyst, however this project was carried using the tire carbon char generated in the process itself.
The project investigates the pyrolysis/gasification of waste tire using a novel catalyst to generate a hydrogen-rich syngas through a gas-solid simultaneous reforming/gasification process. Syngas can be used for hydrogen, power and heat generation. A two-stage pyrolysis-reforming fixed bed reactor was used to carry out the process. The influence of temperature, sample particle size, catalyst and steam addition on hydrogen production were investigated. At the end of each reaction cycle, the pyrolysis contents like gas, liquid and carbon black were weighed and calculated through mass balance equation. The formed liquid fraction was characterized using GC-MS. The obtained oil was compared with different fuel oil to check the validity of pyrolytic oil as a raw material for different chemicals and in an oil feedstock for the petrochemical industry. The obtained gas was analysed using GC. The carbon black was characterized using Scanning electron microscopy (SEM) for morphology of carbon, energy dispersive X-ray analysis (EDX) for elemental composition. A two-stage fixed bed reactor was needed to carry out the process.
Hydrogen-rich syngas and value-added products production form waste gasification